By Victor Smetacek & Adriana Zingone
Originally published in © Nature Magazine , December 5, 2013. Copyright. All text and images courtesy of © Victor Smetacek & Adriana Zingone. All rights reserved.
Sudden beaching of huge seaweed masses smother the coastline and form rotting piles on the shore. The number of reports of these events in previously unaffected areas has increased worldwide in recent years. These ‘seaweed tides’ can harm tourism-based economies, smother aquaculture operations or disrupt traditional artisanal fisheries. Coastal eutrophication is the obvious, ultimate explanation for the increase in seaweed biomass, but the proximate processes that are responsible for individual beaching events are complex and require dedicated study to develop effective mitigation strategies. Harvesting the macroalgae, a valuable raw material, before they beach could well be developed into an effective solution.
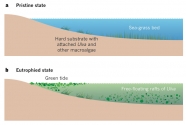
Green, brown and red seaweeds lying on the beach are part and parcel of life in many coastal regions. The amount of beached seaweed biomass started to increase along the shores of industrialized countries in the 1970s, and by the 1990s had become a nuisance along many beaches (1,2) when mass-stranding events of macroalgae became known as green tides. During the 2000s the number of reports from new locations all over the world increased further, as did the magnitude of the beaching events (3). Although non-toxic to humans, seaweed tides harm shore-based activities by virtue of their sheer physical mass. Tonnes of seaweed smothering the shoreline deter tourists and the dense, drifting seaweeds can prevent swimmers and small boats from accessing the sea (Fig. 1); if not removed in time, the algae can turn into a stinking morass, which can produce toxic hydrogen sulphide (H2S) from its anoxic interior1,3, and have major detrimental effects on the affected coastal ecosystems (1,2,4–6.)
Surprisingly, the extensive seaweed tides are mainly the result of only a few genera of macroalgae. Two genera are especially prominent. Species of the genus Ulva, which now includes the former genus Enteromorpha (7), are mainly responsible for green tides. The thallus (vegetative body) is only one or two cells thick but the shapes vary even within spe- cies and can be sheet-like, tubular or fern-shaped (8).
Sargassum — from which the Sargasso Sea takes its name — is the other genus; and we suggest the term ‘golden tide’ to describe the massive shoaling events it is responsible for (after the apt description of floating Sargassum as “The golden floating rainforest of the Atlantic Ocean” (9).
The Sargassum thallus is leathery, tough and differentiated into features that resemble leaves and a stem, and has well-developed gas bladders for flotation. Both Ulva and Sargassum are cosmopolitan, exceptionally species- rich genera and increase their growth rate in response to nutrients (10,11). Whereas most species will only grow when attached to a hard substrate, a few can substantially increase their biomass in a free-floating state, either by increasing the size of the thalli and their fragments, or by making new floating thalli. This is crucial (discussed later) because it is the unattached forms that, by invading new space (the water column), are able to increase their nutrient supply, free themselves from competition for limited hard substrates and avoid their many benthic grazers. As a result, unattached forms can build up large biomasses, forming the massive seaweed tides we discuss in this Perspective. Green tides have occurred all over the world, whereas golden tides have been restricted to beaches between the Gulf of Mexico and Bermuda; however, they significantly increased their range during a spectacular 2011 event.
Ulva green tides
The increase in Ulva biomass on European and US beaches that began (1) in the 1970s was linked to coastal eutrophication. As the many harmful effects became evident, the countries affected took measures to reduce nutrient input to the sea from agricultural sources and sewage. A decline in nutrient concentrations resulted in abatement of the prob- lem in the southern North Sea (12). In other regions, particularly along the popular tourist beaches of Brittany, the magnitude of green tides has been increasing since the 1970s (13).
“The occurrence and magnitude of green tides often vary both annually and seasonally…”
— Victor Smetacek & Adriana Zingone
Beached seaweed has traditionally been collected and used as fertilizer by local farmers, but by the 1990s it had to be taken away by the truckload (Fig. 1). In 2009, H2S gas from Ulva rotting on a Brittany beach caused the death of a horse, and, in 2011, the death of around 30 wild boars. Both incidents were widely reported in the press with some headlines giving the impression that the algae were toxic. The resulting effect on tourism caused severe losses to the local economy, in addition to the costs of removing and disposing the 100,000 tonnes of beached algae (estimated to be US$10–150 per tone) (13).
Increases in the accumulation of Ulva biomass coincided with the expansion of factory livestock farming in Brittany. The consensus among the scientific community seems to be that eutrophication from the effluents of intensive stock rearing is the cause of the increase in number and magnitude of green tides since the 1990s (13). The meat- producing and tourist industries are both mainstays of the provincial economy, and, following the animal deaths, confrontation between the two increased (14).
Brittany is a wet region overloaded with nutrients released by the high density of animals — equivalent to those from 50 million people13 — and so eutrophication is inevitable because the manure is not being shipped back to the animal feed producers outside the province. In efforts to make the best out of a situation that is unlikely to change soon, Ulva biomass has been used as a raw material for biogas production, organic fertiliser and as an additive to animal and human food. However, the value barely meets the costs of current methods of algal collection and processing (13).
In 2008, a spectacular green tide invaded, without warning, the beaches of Qingdao — the venue for the sailing events of the Beijing Olympics. Masses of Ulva floated in from the open water of the Yellow Sea and beached a few weeks before the competition was due to start, ensuring prominent coverage by the international media (Fig. 1). A 30-km-long boom was deployed to keep the masses of floating algae out of the bay, and the removal of more than a million tonnes of algae from the beaches involved 10,000 people at an estimated cost to the province of $30 million (15). In addition, aquaculture operations along the shore suffered losses of $100 million (3).
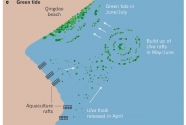
Given the novelty of the phenomenon, the origin, genesis and relationship to eutrophication of the green tide was traced with exemplary speed (16). Analysis of satellite images of the Yellow Sea in 2008 revealed that in total 3,500 km2 were covered with floating algae in patches spread over 84,000 km2. The high nitrate concentrations in the Yellow Sea could explain the high growth rates (21.9% per day) of the floating algal patches (15–17). These patches were subsequently driven inshore by winds, hitting a 140 km stretch of coastline, which included Qingdao. The species responsible, Ulva prolifera, was subsequently shown to proliferate rapidly by sporulation of cells of fragmented thalli; these grew into new thalli by attaching to the parent thallus, with 1–2-mm diameter fragments having the highest sporulation rate (18).
The pelagic seaweed bloom, as well as those in subsequent years, could be traced in satellite images to the coastline some 200 km south of Qingdao where aquaculture of the edible red alga Porphyra yezoensis, which is grown on rafts along the intertidal zone, has expanded rapidly since 2004 (refs 17, 19). Because Ulva prolifera grows profusely on the rafts, thallus fragments dislodged and discarded in the sea during harvesting of Porphyra in spring are the most likely seed source of the mid-summer green tide. If this seeding hypothesis is correct, then collecting and using Ulva as a by-product would mitigate the magnitude of the green tide.
It is estimated that 500 tonnes of Ulva thalli, discarded from the Porphyra rafts, grow into one million tonnes in 6 weeks (15). Another hypothesis based on genetic signatures (5S rDNA spacer sequences), suggests that the Ulva strain responsible for the green tide could be overwintering on the sediment surface south of the Yellow Sea as frag- ments that stem from the summer surface bloom20. The hypotheses are not necessarily mutually exclusive, as the swarm of seeding fragments moving northward from the Porphyra rafts could be augmented by thalli fragments rising to the surface from shallow sediments (Fig. 2). Whatever the source of seeding thalli, green tides along the coasts of the Yellow Sea are recurrent, with the 2013 event reportedly reaching a record level (21).
Managing green tides
The case for a direct connection between spreading coastal eutrophication and the worldwide upsurge in the incidence of green tides is compelling (1,2). However, curbing eutrophication requires significant investment in infrastructure and agricultural practices in the catchment area and can take years to implement, and even longer to take effect. Thus, although water quality in Tokyo Bay has improved, Ulva green tides have increased; the species responsible overwinters as unattached thalli drifting at the sediment surface of shallow waters (22). The notorious Ulva blooms of Venice in the late 1980s, however, are no longer such a nuisance (23,24) even though nutrient concentrations — in particular nitrate — have not significantly diminished in the subsequent decades (24). As biomass accumulation is a function of the seed population multiplied by its growth rate, spreading seed banks of overwintering, free-floating strains of local Ulva could obviate the effects of reducing eutrophication because they are protected from the many Ulva grazers (such as snails and crustaceans) that live on the sea floor (25,26). Thus, green tides have been a chronic problem in the eutrophied northern Baltic since the 1970s — unattached Ulva intestinalis thalli overwinter in shallow, ice-covered waters and rise in the water column, commencing growth in spring (27). Free-floating Ulva species generally persist from spring until autumn in a growth state, after which they transfer the sequestered nutrients to the sediments. In shallow, enclosed seas and fjords these are returned to the surface by vertical mixing (that is, retained within the system) (13). Thus, in favorable topographical and hydrological environments, free-floating macroalgae are likely to continue to proliferate and maintain the eutrophic state that is favourable for their perpetuation.
The occurrence and magnitude of green tides often vary both annually and seasonally, hence the planning of mitigation measures will require interdisciplinary investigation of the life-cycle strategies of the species involved in relation to the physical and chemical setting of the environment: topography, circulation, wind patterns and the nutrient regime, exemplified by the work done on the Yellow Sea green tides (3,15– 20,28–30). An obvious mitigation target would be the overwintering and early spring growing stages. The costs of collecting and disposing of Ulva masses in spring would have to be weighed against their nuisance ‘value’ in the summer.
“During 2011, there was an ocean-scale build-up of Sargassum that at its peak extended across the Atlantic and resulted in massive golden tides along the west African coast, from Sierra Leone to Ghana, and, on the other side of the Atlantic, from Trinidad to the Dominican Republic…”
— Victor Smetacek & Adriana Zingone
In the Yellow Sea, the profit from Porphyra aquaculture amounts to $53 million, whereas the cost of removing Ulva from the beaches is estimated to be $30 million15. As elsewhere, the overburdened local governing bodies are responsible for keeping their beaches clean. In Brittany, the tourism industry ($5.1 billion) and the farming sector ($11.6 billion) have been pitted against each other (31,32). Recently, policy makers at national and European Union levels have passed measures to curb factory farming practices, which has led to closures, lay-offs and protests in the area (33). Similar tensions have begun to arise in China between the Jiangsu province, home to Porphyra aquaculture, and Shandong province, whose coasts have been affected by green tides (34).
Sargassum golden tides
Golden tides due to the beaching of floating Sargassum occur regularly in summer along the coasts of the Gulf of Mexico and are often a nuisance on tourist beaches (35). An increase in golden tides during the 1980s and 1990s has been linked to higher nutrient loads of the Mississippi river (10). However, compared with Sargassum in the Sargasso Sea — often referred to as “the only sea without a coastline” (9) — little is known about Sargassum in the Gulf of Mexico. Analysis of satellite images from 2002 to 2008 revealed that floating Sargassum originated in the north-western Gulf of Mexico each spring and was exported to the Sargasso Sea where it accumulated in the summer months and by winter had disappeared36, presumably because aged thalli sank to the deep sea (37). From satellite images, an estimated one million tonnes wet weight of Sargassum is exported to the Atlantic each year (36). For comparison, about the same mass of Ulva was collected and disposed of on land during the green tide of the 2008 Olympics (3), and a similar amount was estimated to have accumulated in the Venice lagoon during the peak outbreak (23).
Floating Sargassum, represented by the two species Sargassum natans and Sargassum fluitans, is deemed a valuable and unique habitat that harbours many highly adapted, and even endemic animal species that depend on Sargassum for food (9). If Sargassum is imported to the Sargasso Sea each year, it is hard to imagine how the dependent animal species, which beach with the Sargassum, could have evolved with such a ‘one-way’ seasonal life cycle. Nevertheless, the satellite observations are supported by ship-based reports of the seasonal cycle of floating Sargassum (36). Thus, the processes maintaining the floating Sargassum habitat through the winter and the specific properties of the north- western Gulf that allow rapid growth of new thalli during spring, warrant investigation.
S. natans and S. fluitans are considered to be the only holopelagic macroalgae, so why is a specific geographical seeding site part of their life cycle? This question gains importance because of the commercial interest in Sargassum biomass; patents have been filed for growing and harvesting Sargassum in the Sargasso Sea. Furthermore, an international alliance of scientists has recently been formed to protect and manage the Sargasso Sea (9).
During 2011, there was an ocean-scale build-up of Sargassum that at its peak extended across the Atlantic and resulted in massive golden tides along the west African coast, from Sierra Leone to Ghana, and, on the other side of the Atlantic, from Trinidad to the Dominican Republic (Fig. 3) (38). The peak biomass during the 2011 event was 200- fold higher than the previous 8 years’ average biomass peak recorded in the region (39). According to eyewitnesses, beached Sargassum was unknown in northwest Africa before 2011, so the event came as a shock to the many afflicted fishing villages, and has been attributed to the effects of offshore oil production that had started at the time (40). The afflicted southern Caribbean islands had never, within living memory, experienced an event of this magnitude (38). Satellite images showed that the algal rafts had developed along the northern coast of Brazil, north of the mouth of the Amazon, from where they moved east and west, eventually stretching from shore to shore (Fig. 3) (36). The effects on the beaches were substantial; however, because no popular tourist beaches or big cities were affected the African events were only reported in regional media. Along the western coast of Ghana a blanket of Sargassum extended for kilometres offshore, clogging fishing nets and impeding the passage of small boats. This resulted in food shortages for people living in villages dependent on artisanal fisheries for their livelihood (41). In the Caribbean, tourism was affected because of the closure of beaches and bays (Fig. 1).
Unfortunately, the satellite sensor from which the images in Fig. 3 were produced (MERIS) went out of service in early 2012 (ref. 39). We could not find any reports of unusually large golden tides in subsequent years from either the southern Caribbean islands or the west coast of Africa.
Explaining the unprecedented, ocean-scale build-up of Sargassum biomass in 2011 will require much detective work by physical, chemical and biological oceanographers because its occurrence challenges the current concept that the Sargasso Sea is a closed system (9).
Indeed, an unanswered question raised in the 1970s asked why there are five subtropical ocean gyres (STG) but only one, the North Atlantic, harbours the Sargasso Sea. The circulation pattern of all STGs is essentially similar and all impinge on extensive coastlines along their western flanks. The long, unbroken evolutionary history of floating Sargassum is evidenced by the many adaptations, in particular perfect camouflage, that various animal classes have evolved in response to life in this floating habitat.The highly specialized sargassum fish (Histriohistrio) has a pan-tropical distribution (including the west African coast) (42), prompting the questions: does Sargassum exist in large enough quantities to provide a habitat for specialized species in other STGs and how could this change in the future. Was the 2011 Sargassum bloom a freak event caused by a unique collection of non-linear environmental factors impinging on each other to create an environment in which Sargassum thrived? Or does it represent a symptom of an ocean-wide response to increasing pressure on the biosphere from anthropogenic waste, and hence an indication of what is to come?
Establishing an international consortium
Blooms of noxious phytoplankton (unicellular microalgae), known as red tides and later as harmful algal blooms (HABs), occur in coastal regions worldwide. In most cases, the harmful effects are caused by phytoplankton species that render seafood toxic, result in the mass death of marine animals or affect aquaculture operations. High-biomass phy- toplankton blooms can also be a nuisance on beaches by discolouring the water (red and brown tides) or forming scums and foams (43). Their biomass is low compared with green tides, but they can also cause anoxic events, albeit in deeper water. HAB research has substantially increased over the past decades, greatly profiting from international organisations such as the Global Ecology and Oceanography of Harmful Algal Blooms programme (IOC–SCOR GEOHAB) (44). The seaweed blooms discussed in this Perspective are very different (2) and only have two features in common with microalgal HABs: they grow in a free-floating state (that is, they compete with phytoplankton for nutrients) and cause harm to the affected coastlines. Because they are essentially benthic organisms that live a planktonic existence, the investigation of free-floating macroalgae will need to combine established research methods from both fields, and apply new techniques for surveying, sampling and modelling them. It would be advisable to develop such a dedicated, interdisciplinary research programme at an international level.
The focus of this scientific network could be the comparatively few species of macroalgae that can all increase biomass in a free-floating stage, either drifting above the bottom in shallow waters, or floating at the surface further out at sea. Most green-tide species belong to the former category (1,2) and, to our knowledge, the latter has only been reported in the Yellow Sea. The surface-floating Ulva prolifera strain has been shown to differ from attached Ulva species of the Yellow Sea coastline (15).
“…in-depth understanding of the growth dynamics of massive seaweed tide species is not only a prerequisite for developing cost-effective mitigation strategies, but it could also provide the basic knowledge required to manage free-floating algae as a potentially valuable resource.”
— Victor Smetacek & Adriana Zingone
Is this surface-floating form a new genotype that can only attain massive biomass in settings with the hydrographic features and wind patterns of the Yellow Sea? Or are other shallow seas, such as the North Sea, susceptible to invasion by a surface-floating form of Ulva? This could happen either by evolution within the local species pool or by inadvertent introduction of the Yellow Sea form. Among the questions that need to be addressed is the buoyancy regulation mechanism of free-floating Ulva, which allows thalli to stay suspended in the water column or rise to the surface. Furthermore, life cycles also need to be studied in situ. The ability to produce new thalli on the surface of the parent thallus (18) suggests the absence of chemical deterrents, which are known to exist for the thallus surface of other Ulva species (45,46). In the case of Sargassum, the factors necessary for large-scale spring regeneration of thalli require elucidation, as well as hindcasting for the factors that allowed the 2011 Sargassum event. Finally, the fact that surface-floating Ulva and Sargassum rafts were able to proliferate in the open sea indicates their ability to compete with phytoplankton for nutrients. Biomass build-up of dense macroalgal clumps, in contrast to diffuse phytoplankton, is presumably enabled by wind energy pushing the rafts through the water, which can vastly increase their nutrient supply. One wonders why evolution of this surface-floating macroalgal life form in the open sea is restricted to so few genera.
Mitigation or amelioration?
An in-depth understanding of the growth dynamics of massive seaweed tide species is not only a prerequisite for developing cost-effective mitigation strategies, but it could also provide the basic knowledge required to manage free-floating algae as a potentially valuable resource.
Ulva biomass contains a number of compounds of interest to the food-additive industry, and a biorefinery plant to process the Ulva biomass collected from the beach or shallow water has recently been established in the region of Brittany plagued by green tides (47). One of the aims is to provide an alternative food additive to the fish-meal-based one currently given to farmed fish. In order for this to work, the costs of collecting Ulva need to be similar to those of collecting fish used to make fish meal. In the case of surface-floating Ulva, ‘catching’ the algae at sea by making use of ships from the current fishing fleet, which are already equipped with the facilities required to preserve the catch, should be competitive in terms of cost. Patches of floating rafts located by aerial surveys could be concentrated with booms similar to those used for containing oil spills, and the thalli pumped on deck and collected on nets and filters of decreasing mesh size (to collect fragments). If carried out in the early stages of the bloom, this technique could mitigate the magnitude of the beaching events.
In other regions, techniques to collect Ulva masses in shallow water from special ships by rakes, nets or suction pipes could be developed and one technique is already in operation in the Venice lagoon (23). Transporting the Ulva biomass to processing factories from harbours should be much cheaper and easier than using rakes and tractors on beaches; the quality of the raw material will also be superior because it will be fresher and contain less sand. Price depends on demand, so encouraging the establishment of Ulva-processing factories will raise the price of the raw material — and could well make floating rafts of Ulva an interesting target for a new, summer fishery. In the case of Sargassum, also a valuable raw material, harvesting by ship is already regulated in the western Atlantic (48). Needless to say, harvesting floating macroalgae is the logical and ultimate (49) step in the process known as “fishing down marine food webs”.
It should also be pointed out that the carbon-to-nitrogen ratio of oceanic Sargassum is around 50:1 (ref. 10) and the alga’s tendency to rapidly sink to the deep-sea floor makes it a much more efficient vehicle to artificially sequester carbon in the oceans than phytoplankton, which have carbon-to-nitrogen ratios of less than 10:1.
The future impact of green and golden tides could be very different if they become regarded as potential crops rather than harmful weeds.
References
- 1. Fletcher, R. T. in Marine Benthic Vegetation – Recent Changes and the Effects of Eutrophication (eds Schramm, W. & Nienhuis, P. H.) 7–43 (Springer, 1996).
- 2. Valiela, I. et al. Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol. Oceanogr. 42, 1105–1118 (1997).
- 3. Ye, N. H. et al. ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol. Res. 26, 477–485 (2011).
- 4. Norkko, A. & Bonsdorff, E. Population responses of coastal zoobenthos to stress induced by drifting algal mats. Mar. Ecol. Prog. Ser. 140, 141–151 (1996).
- 5. Norkko, A. & Bonsdorff, E. Rapid zoobenthic community responses to accumulations of drifting algae. Mar. Ecol. Prog. Ser. 131, 143–157 (1996).
- 6. Arroyo, N. L., Aarnio, K., Mäensivu, M. & Bonsdorff, E. Drifting filamentous algal mats disturb sediment fauna: Impacts on macro–meiofaunal interactions. J. Exp. Mar. Biol. Ecol. 420–421, 77–90 (2012).
- 7. Hayden, H. S. et al. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 38, 277–294 (2003).
- 8. Blomster, J. et al. Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. Am. J. Bot. 89, 1756–1763 (2002).
- 9. Laffoley, D. A. et al. The Protection and Management of the Sargasso Sea: The Golden Floating Rainforest of the Atlantic Ocean 1–44 (Washington, 2011).
- 10. Lapointe,B.E.A comparison of nutrient-limited productivity in Sargassum natans from neritic vs. oceanic waters of the western North Atlantic Ocean. Limnol. Oceanogr. 40, 625–633 (1995).
- 11. Teichberg,M.etal. Eutrophication and macroalgal blooms intemperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Glob. Change Biol. 16, 2624–2637 (2010).
- 12. van Beusekom,J.E. E.etal. Quality Status Report 2009. Wadden Sea Ecosystem No. 25 (eds Marencic, H. & de Vlas, J.) 1–21 (Common Wadden Sea Secretariat, Trilateral Monitoring and Assessment Group, 2009).
- 13. Charlier,R.H.,Morand,P.&Finkl,C.W.How Brittany and Florida coast scope with green tides. Int. J. Environ. Stud. 65, 191–208 (2008).
- 14. Saltmarsh,M. A battle between economic mainstays in Brittany. (New York Times, 2010).
- 15. Liu, D.etal. The world’s largest macroalgal bloom in the Yellow Sea,China: formation and implications. Estuar. Coast. Shelf Sci. 129, 2–10 (2013).
- 16. Sun,S.etal. Emerging challenges: Massive green algae blooms in the Yellow Sea. Nature Preced.(2008).
- 17. Keesing,J.K.,Liu,D.,Fearns,P.& Garcia,R.Inter-andintra-annual patterns of
Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar. Pollut. Bull. 62, 1169–1182 (2011). - 18. Gao,S.etal.A strategy for the proliferation of Ulva prolifera, main causative species of green tides, with formation of sporangia by fragmentation. PLoS ONE 5, e8571 (2010).
- 19. Hu, C. et al. On the recurrent Ulva prolifera blooms in the Yellow Sea and East China Sea. J. Geophys. Res. 115, C05017 (2010).
- 20. Liu, F. et al. Understanding the recurrent large-scale green tide in the Yellow Sea: temporal and spatial correlations between multiple geographical, aquacultural and biological factors. Mar. Environ. Res. 83, 38–47 (2013).
- 21. Jacobs, A. With surf like turf, huge algae bloom befouls China coast. (New York Times, 2013).
- 22. Yabe,T.etal.Green tide formed by free-floating Ulva spp.at Yatsu tidal flat,
Japan. Limnology 10, 239–245 (2009). - 23. Sfriso, A. & Marcomini, A. Decline of Ulva growth in the lagoon of Venice.
Bioresour. Technol. 58, 299–307 (1996). - 24. Facca,C.,Pellegrino,N.,Ceoldo,S.,Tibaldo, M.&S friso,A. Trophic conditions in
the waters of the Venice lagoon (Northern Adriatic Sea, Italy). Open Oceanogr. J.
5, 1–13 (2011). - 25. Geertz-Hansen,O.,Sand-Jensen,K.,Hansen,D.F.&Christiansen,A.Growth
and grazing control of abundance of the marine macroalga, Ulva lactuca L. in a
eutrophic Danish estuary. Aquat. Bot. 46, 101–109 (1993). - 26. Kamermans,P.etal.Effect of grazing by isopods and amphipods in growth of
Ulva spp. (Chlorophyta). Aquat. Ecol. 36, 425–433 (2002). - 27. Bäck,S.,Lehvo,A.&Blomster,J.Mass occurrence of unattached Enteromorpha
intestinalis on the Finnish Baltic Sea coast. Ann. Bot. Fenn. 37, 155–161 (2000). - 28. Lin,H.Z.etal. Genetic and marine cyclonicaldy analyses on the largest
macroalgal bloom in the world. Environ. Sci. Technol. 45, 5996–6002 (2011). - 29. Zhang, X. W. et al. Somatic cells serve as a potential propagule bank of
Enteromorpha prolifera forming a green tide in the Yellow Sea, China. J. Appl.
Phycol. 22, 173–180 (2010). - 30. Zhang, J. H. et al. Growth characteristics and reproductive capability of green
tide algae in Rudong coast, China. J. Appl. Phycol. 25, 795–803 (2013). - 31. Viscusi, G.
Fear of noxious ‘green tides’ drives tourists from beaches of Brittany(Boston Globe, 2011). - 32. Diaz,M., Darnhofer,I., Darrot,C.& Beuret,J.-E.Green tides in Brittany: What
can we learn about niche–regime interactions? Environ. Innov. Soc. Transitions 8,
62–75 (2013). - 33. Samuel,H.
French protesters say Brittany will be François Hollande’s‘cemetery’ (The Telegraph, 2013). - 34. Jing,L. Seaweed farming linked to Qingdao’s green tide of algae (South China Morning Post, 2013).
- 35. Williams, A.& Feagin,R.Sargassum as a natural solution to enhance dune plant growth. Environ. Manage. 46, 738–747 (2010).
- 36. Gower,J.& King,S.Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic Ocean mapped using MERIS. Int. J. Remote Sens. 32, 1917–1929 (2011).
- 37. Johnson,D.L.& Richardson,P.L.On the wind-induced sinking of Sargassum. J. Exp. Mar. Biol. Ecol. 28, 255–267 (1977).
- 38. Hemphill,A. Change is in the air–seaweed, seaweed everywhere! (Arlo Hemphill, 2013).
- 39. Gower,J.,Young,E.&King,S.Satellite images suggest a new Sargassum source region in 2011. Remote Sens. Lett. 4, 764–773 (2013).
- 40. Ackah-Baidoo,A.Fishing in troubled waters: oil production, seaweed and community-level grievances in the Western Region of Ghana. Community Dev. J. 48, 406–420 (2013).
- 41. McDiarmid,J.Western Ghana’s fisher folk starve amid algae infestation (IPS, 2011).
- 42. Froese,R.&Pauly,D.(eds). FishBase.(Fishbase, 2013).
- 43. Anderson,D.M.,Cembella,A.D.&Hallegraeff,G.M.Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 4, 143–176 (2012).
- 44. GEOHAB.Global Ecology and Oceanography of Harmful Algal Blooms (SCORand IOC, 2001).
- 45. Nelson,T.A.,Lee,D.J.& Smith,B.C. Are “green tides” harmful algal blooms? Toxic properties of water-soluble extracts from two bloom-forming macroalgae, Ulva fenestrata and Ulvaria obscura (Ulvophyceae). J. Phycol. 39, 874–879 (2003).
- 46. Harder,T., Dobretsov,S.&Qian,P.-Y.Water born epolarmacromoleculesact as algal antifoulants in the seaweed Ulva reticulata. Mar. Ecol. Prog. Ser. 274, 133–141 (2004).
- 47. Algae Industry Magazine. Olmix opens algae biorefinery in Brittany(Algae Industry Magazine, 2013).
- 48. South Atlantic Fishery Management Council. Fishery Management Plan For Pelagic Sargassum Habitat Of The South Atlantic Region(NOAA, 2002).
- 49. Pauly,D.,Christensen,V.,Dalsgaard,J.,Froese,R.&Torres,F.Fishingdown marine food webs. Science 279, 860–863 (1998).